Real-World Applications of Chemistry
What do Chemistry Cat, toilet bowl cleaner and contact lens cleaning solution have in common? They’re all ways to take the abstract subject of chemistry and make it come alive and make sense for high school students. Throughout my career, I have strived to make this subject applicable to the real world, and I’d like to share some labs I have students do to make that happen.
First, there’s the toilet bowl cleaner lab. (“Really? You do a lab about toilet bowl cleaners?” Yep!) It reveals interesting things about branding and marketing of consumer products. To begin, you need several brands of toilet bowl cleaners that use hydrochloric acid as the main cleaning agent. (Cleaners that use organic surfactants won’t work here.) I use three main brands: Lysol®, Sno Bol® and The Works®. Not only do these cleaners vary in cost per mL, but they also vary in effectiveness, as students soon discover. The main concept behind this lab is for students to perform several microscaled titrations and determine the acid concentration in each cleaner. I have students use a given volume of cleaner (10 drops using a standard plastic pipette), a few drops of phenolphthalein indicator and 3 mL of water (to add some volume to the solution, as toilet bowl cleaner can be viscous). They then add the correct number of drops of 1.0 molar sodium hydroxide solution until the acid in the cleaner has been neutralized and the phenolphthalein indicator goes from colorless to faint pink. Using the relationship Macid x Vacid = Mbase x Vbase, students calculate the concentration of acid in each cleaner. Not only do they find that the off-brand cleaner, The Works®, is the most cost effective in terms of cost per mL, they see that it has a much higher concentration of acid than either Sno Bol® or Lysol®. Knowledge of chemistry really can save you a lot of money!
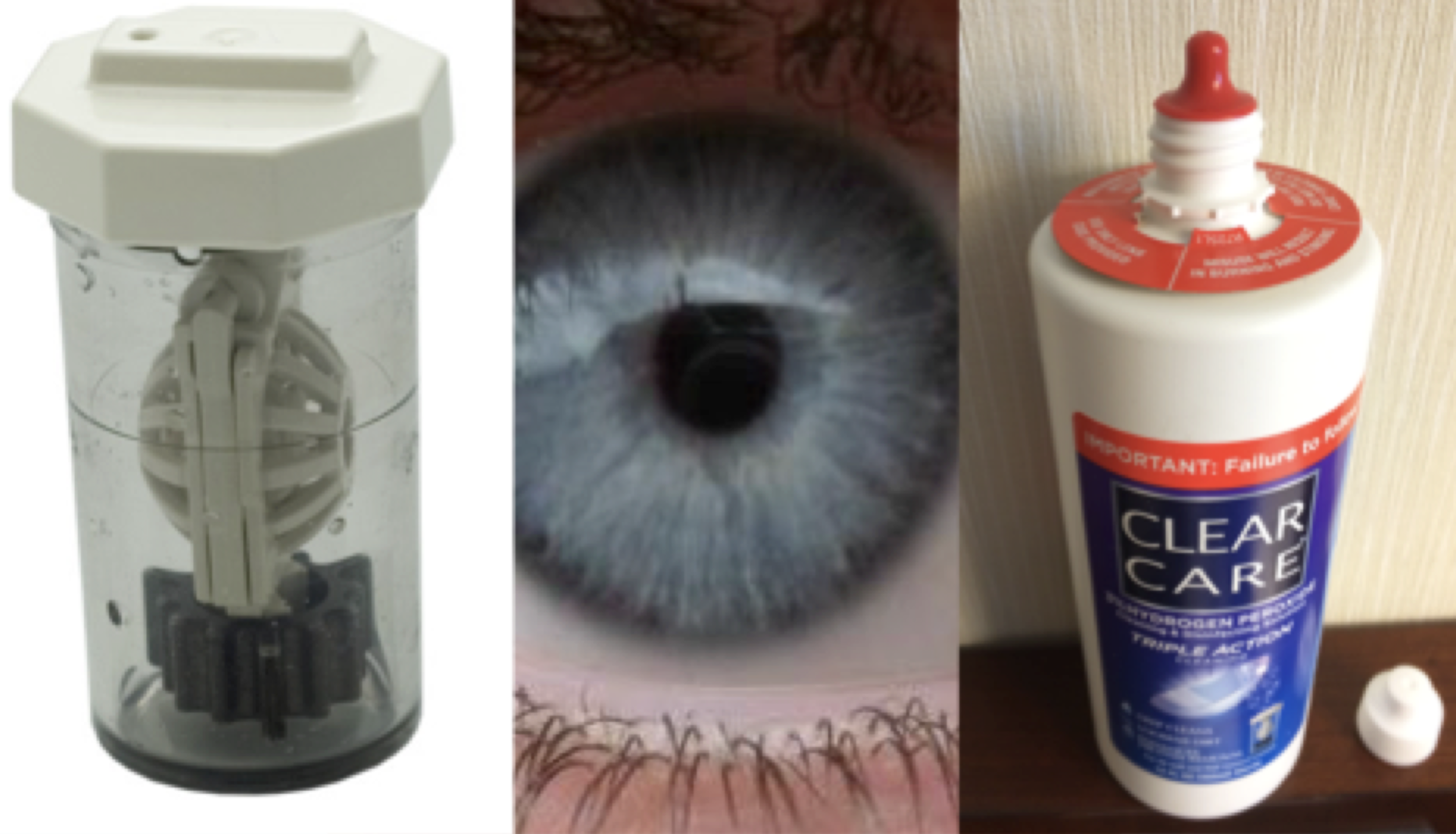
The second lab I’d like to share — from a 2003 Journal of Chemical Education article (volume 80, issue 7, page 788) — uses a hydrogen peroxide–based cleaner (hence the Chemistry Cat meme). The cleaner (like CLEAR CARE® from Alcon) is put into a plastic container with a platinum disc to catalyze the reaction of hydrogen peroxide to become water and oxygen gas. According to the manufacturer, contact lenses must be placed in a plastic case and soaked in the peroxide solution for a period of six hours. At that point, the concentration of peroxide is so low that if someone were to put the lenses back in their eyes without rinsing with a saline solution it would still be pretty safe. (You are basically just using a solution of oxygenated water at this point, which is not harmful to the eye.)
The problem with this cleaning solution is that several consumers confuse it with other clean-and-rinse multipurpose contact lens solutions and put full strength CLEAR CARE® straight into their eyes. On misusing this product, one consumer said, “I jammed my 24-year-old daughter’s eye under the faucet. Her eyes were burning for a couple of days.” Another said, “BURNING. My eye was bright red for pretty much the next 24 hours, to the point that I refused to do anything social that didn’t include sunglasses. I’m so paranoid about solution now.”
The lesson of this experiment is that students use kinetic concepts to determine the actual amount of time needed for the hydrogen peroxide concentration to go from full strength to a safe, acceptable level. When they conclude from their data that it only takes three hours, instead of the suggested time of six hours, they realize that chemistry, again, helps them see the truth behind the product.
My advice: Help your students make those connections to real-world, consumer applications.
About The Author: About the Author: Ray Lesniewski, a National Board Certified educator, has taught fifth grade through college-level chemistry since 1987. Along with a master’s degree in chemistry, he has an extensive background in theatre, voice-over work and choral music. Ray tries to integrate the performing arts whenever possible to help make chemistry concepts come alive for his students.
*All
trademarks are the property of their respective owners and are not affiliated
with, nor endorsed by Texas Instruments.
Tagcloud
Archive
- 2026
- 2025
- 2024
- 2023
- 2022
-
2021
- January (1)
- February (3)
- March (5)
-
April (7)
- Top Tips for Tackling the SAT® with the TI-84 Plus CE
- Monday Night Calculus With Steve Kokoska and Tom Dick
- Which TI Calculator for the SAT® and Why?
- Top Tips From a Math Teacher for Taking the Online AP® Exam
- Celebrate National Robotics Week With Supervised Teardowns
- How To Use the TI-84 Plus Family of Graphing Calculators To Succeed on the ACT®
- AP® Statistics: 6 Math Functions You Must Know for the TI-84 Plus
- May (1)
- June (3)
- July (2)
- August (5)
- September (2)
-
October (4)
- Transformation Graphing — the Families of Functions Modular Video Series to the Rescue!
- Top 3 Halloween-Themed Classroom Activities
- In Honor of National Chemistry Week, 5 “Organic” Ways to Incorporate TI Technology Into Chemistry Class
- 5 Spook-tacular Ways to Bring the Halloween “Spirits” Into Your Classroom
- November (4)
- December (1)
-
2020
- January (2)
- February (1)
- March (3)
- April (1)
- May (2)
- July (1)
- August (2)
- September (3)
-
October (7)
- Tips for Teachers in the time of COVID-19
- Top 10 Features of TI-84 Plus for Taking the ACT®
- TI Codes Contest Winners Revealed
- Best of Chemistry Activities for the Fall Semester
- Best of Biology Activities for the Fall Semester
- Best of Physics Activities for the Fall Semester
- Best of Middle Grades Science Activities
- November (1)
- December (2)
- 2019
-
2018
- January (1)
- February (5)
- March (4)
- April (5)
- May (4)
- June (4)
- July (4)
- August (4)
- September (5)
- October (8)
-
November (8)
- Testing Tips: Using Calculators on Class Assessments
- Girls in STEM: A Personal Perspective
- 5 Teachers You Should Be Following on Instagram Right Now
- Meet TI Teacher of the Month: Katie England
- End-of-Marking Period Feedback Is a Two-Way Street
- #NCTMregionals Kansas City 2018 Recap
- Slope: It Shouldn’t Just Be a Formula
- Hit a high note exploring the math behind music
- December (5)
- 2017
- 2016
- 2015
