The Best Equilibrium Simulation Ever
Chemical equilibrium is one of the most difficult concepts for students to understand, and it’s even more difficult to find a model that will work every time. Like the late-night infomercials say, “I’ve tried everything.” I’ve used the popular method of moving water from one beaker to another using two straws of different widths. This approach can result in always-changing water levels due to spillage. Students don’t get to see that equilibrium means that there is a constant rate of change between two parts of a system and that, at equilibrium, you usually get different, yet constant, levels of water in both beakers. There have been other models, too, involving nuts and bolts, film cans with and without lids, and blindfolds, but none of these methods give good, reasonable, quantifiable results.
Eventually, I found a system from the Connected Chemistry Curriculum (found at connchem.org) that is interesting, robust with meaning, and foolproof. All we need is a few hundred pennies (or buttons, M&M’S®, etc.). Students start out with 40 reactant pennies and zero product pennies. They move the pennies from the reactant to the product side and back with just a few simple rules: move ½ of the reactant pennies to the product side and then move ¼ of the product pennies to the reactant side; any time students end up with a fractional amount of pennies, they must round that up to the nearest whole number. Then they count the new number of pennies on each side, recording the data and continuing with another round. Keep these rounds going until students believe they are at equilibrium. The results are very reliable, and there are many interesting variations. Here’s what students see when the results are graphed for the experiment I just described.
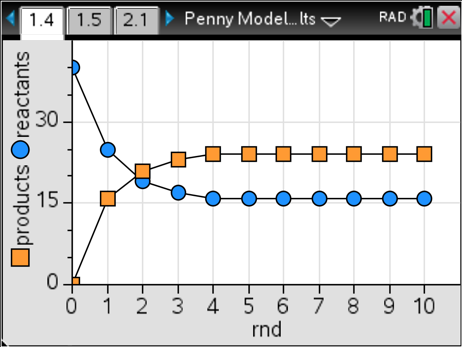
There are a few things that are great about this model: 1) It always gives good results; 2) The reactant and product amounts are not equal to each other, yet their numbers are constant starting in round four; and 3) Students can try this out with different starting amounts and will still get constant amounts at equilibrium. For example, here’s what happens when starting with four reactant pennies and 36 product pennies.
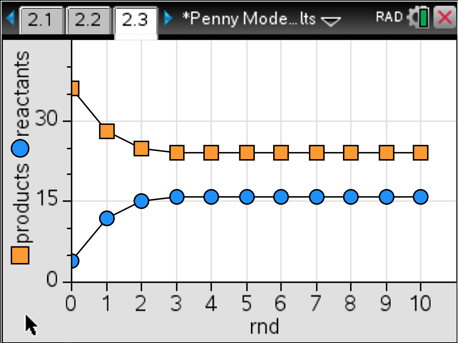
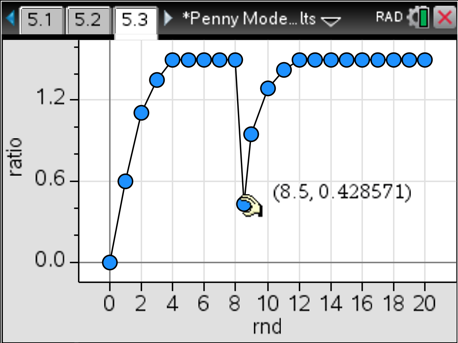
In this example, we reach equilibrium one round earlier than in the last case and find that there are different amounts of reactants and products compared to the first case. Here’s where it gets really good. We calculate the ratio of products to reactants in each round for each simulation, and graph that. Here’s what we get for cases #1 and #2.
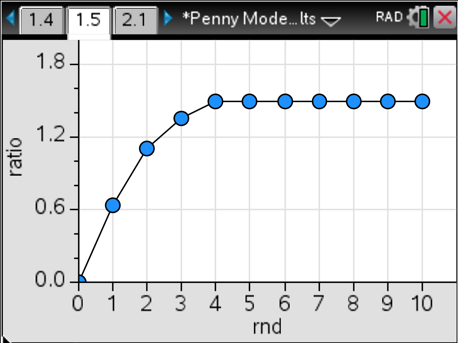
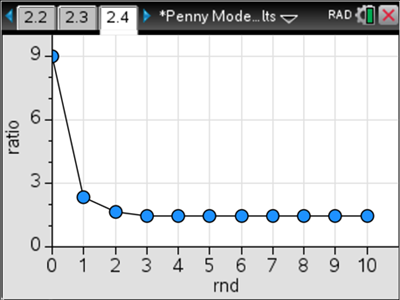
In both cases, we end up with a ratio of 1.5 product pennies for every one reactant penny at equilibrium. That’s pretty powerful and is directly applicable to the chemistry behind systems in equilibrium. What I like is that we have a concrete and reliable physical model that students can actually see, manipulate and analyze.
Here’s the best part: We can then extend this model to illustrate Le Chatelier’s Principle. Students establish a system in equilibrium using case #1 and then stress it by adding 20, 30 or 40 more pennies to the system. The results are beautiful. In all three cases, we reach a new equilibrium with different amounts of products compared to reactants, yet the ratio is always 1.5. That’s exactly what we want to see. Here’s what the graphs look like for a stress of 40 extra pennies added between rounds eight and nine.
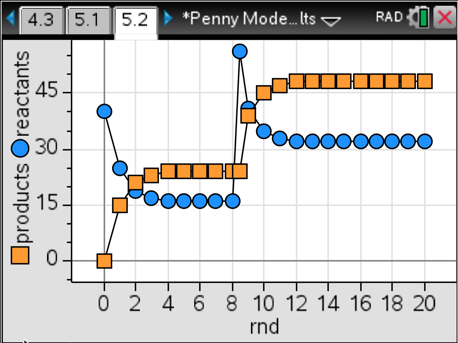
I like to have different groups use different stresses and then compare and contrast their graphs. There’s a lot of powerful learning that takes place with this model while always giving dependable results using a concrete example. I like to refer back to this physical model when we are studying abstract chemical systems, and the light bulbs always come on in their heads. Give it a try.
AP® is a trademark registered by the College Board, which is not affiliated with, and does not endorse, TI products. Policies subject to change. Visit www.collegeboard.com.
M&M’S is a registered trademark of its owner.
About the author: Ray Lesniewski, a National Board Certified educator, has taught fifth grade through college-level chemistry since 1987. Along with a master’s degree in chemistry, he has an extensive background in theatre, voice-over work and choral music. Ray tries to integrate the performing arts whenever possible to help make chemistry concepts come alive for his students.
Tagcloud
Archive
- 2026
- 2025
- 2024
- 2023
- 2022
-
2021
- January (1)
- February (3)
- March (5)
-
April (7)
- Top Tips for Tackling the SAT® with the TI-84 Plus CE
- Monday Night Calculus With Steve Kokoska and Tom Dick
- Which TI Calculator for the SAT® and Why?
- Top Tips From a Math Teacher for Taking the Online AP® Exam
- Celebrate National Robotics Week With Supervised Teardowns
- How To Use the TI-84 Plus Family of Graphing Calculators To Succeed on the ACT®
- AP® Statistics: 6 Math Functions You Must Know for the TI-84 Plus
- May (1)
- June (3)
- July (2)
- August (5)
- September (2)
-
October (4)
- Transformation Graphing — the Families of Functions Modular Video Series to the Rescue!
- Top 3 Halloween-Themed Classroom Activities
- In Honor of National Chemistry Week, 5 “Organic” Ways to Incorporate TI Technology Into Chemistry Class
- 5 Spook-tacular Ways to Bring the Halloween “Spirits” Into Your Classroom
- November (4)
- December (1)
- 2020
- 2019
-
2018
- January (1)
- February (5)
- March (4)
- April (5)
- May (4)
- June (4)
- July (4)
- August (4)
- September (5)
- October (8)
-
November (8)
- Testing Tips: Using Calculators on Class Assessments
- Girls in STEM: A Personal Perspective
- 5 Teachers You Should Be Following on Instagram Right Now
- Meet TI Teacher of the Month: Katie England
- End-of-Marking Period Feedback Is a Two-Way Street
- #NCTMregionals Kansas City 2018 Recap
- Slope: It Shouldn’t Just Be a Formula
- Hit a high note exploring the math behind music
- December (5)
- 2017
- 2016
- 2015
