Molecular Titration
TI-Nspire™ CX CAS
Molecular Titration
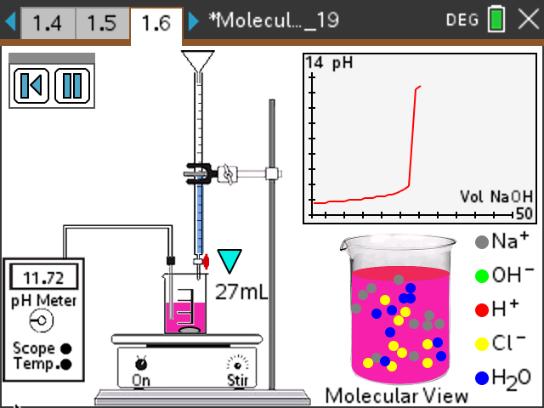
In this lesson, students explore a simulation of a pH titration that includes a molecular view of the chemical changes that occur as a strong base (NaOH) is added to a beaker containing a strong acid (HCl) solution.
- Students will observe what happens during a titration of a strong acid with a strong base, using a simulation accompanied by a molecular view and pH graph.
- Students will determine the volume of base needed to reach the equivalence point.
- Students will see how pH is related to an excess of H+ ions or an excess of OH– ions in a solution.
- acid dissociation constant
- aliquot
- concentration
- equivalence point
- pH
- strong acid
- strong base
- titration
This lesson features a simulation of a pH titration that includes a molecular view of the chemical changes that occur as a strong base (NaOH) is added to a beaker containing a strong acid (HCl) solution.
As a result, students will have a better understanding of:
- The nature of strong acids and strong bases.
- The chemical species present before, after, and at the equivalence point.
This activity was created using Lua. Learn more about Lua in TI-Nspire here.
TI-Nspire™ CX CAS
Vernier EasyData,Vernier EasyLink and Vernier EasyTemp are registered trademarks of Vernier Science Education.

