Light Me Up!
Light Me Up!
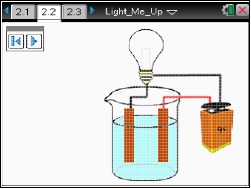
In this lesson, students will explore from a macroscopic level what actually occurs on the microscopic level when electrodes and a source of DC electricity are placed in a solution.
- Students will observe whether different solutions conduct electricity.
- Students will relate this information to the concept of electrolytes and nonelectrolytes.
- Student will make predictions as to whether a solution will conduct electricity well, poorly, or not at all.
- Students will use solubility rules to predict the conductivity of ionic compounds.
- anions
- cations
- conductivity
- covalent compound
- direct current (dc)
- dissociation
- electrolytes
- ionic compound
- ionization
- molecules
- nonelectrolytes
- solubility
- strong electrolyte
- weak electrolyte
This lesson shows on the macroscopic level what occurs on the microscopic level when electrodes and a source of DC electricity are placed in a solution.
As a result, students will:
- Observe the particles in the solution when a source of current is added.
- Identify electrolytes and nonelectrolytes.
- Predict whether substances will conduct electricity well, poorly, or not at all.
This activity was created using Lua. Learn more about Lua in TI-Nspire here.
Vernier EasyData,Vernier EasyLink and Vernier EasyTemp are registered trademarks of Vernier Science Education.

