Build an Atom (HS)
Build an Atom (HS)
In this lesson, students will simulate, observe, and manipulate the variables that affect the structure and, therefore, the properties of an atom.
- Students will discover that atoms are made up of particles and that the number of particles in an atom affects its properties.
- Students will learn that these particles - protons, neutrons, and electrons - are found in specific locations in the atom.
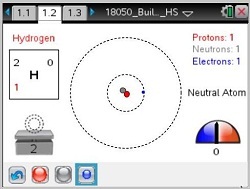
- Students will learn that neutrons have a neutral charge, protons a positive charge (+), and electrons a negative (-) charge; atoms become unstable with large imbalances between the charges.
- Students will learn that an atom is neutral when there is the same number of protons as electrons; the charge of the atom becomes either positive or negative, depending on the net gain of either electrons (-) or protons (+).
- Students will learn about how atomic number, mass and net charge are determined.
- Students will learn about the organization of the Periodic Table.
- proton
- neutron
- electron
- ion
- net charge
Adapted from a PhETTM simulation, lesson involves students manipulating an atom's composition and observing the change in atom identity, stability, overall charge, size, and atomic symbol
PhET is a trademark owned by the Regents of the University of Colorado, which was not involved in the production of, nor do they endorse this product.
Vernier EasyData,Vernier EasyLink and Vernier EasyTemp are registered trademarks of Vernier Science Education.

