Writing Chemical Formulas
Writing Chemical Formulas
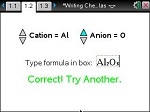
In this lesson, students practice writing chemical formulas for ionic binary compounds.
- Students will understand that the chemical formula identifies each constituent element by its chemical symbol.
- Students will understand that a subscript after the chemical symbol indicates the number of atoms of each element found in each discrete molecule of that compound.
- Students will recognize that the valence of an element determines the ratio that atoms will combine with one another to form a neutral compound.
- Students will understand that atoms always combine with one another in simple whole number ratios.
- cation
- anion
- ionic compound
- ionic charge
- valence
This lesson involves students practicing writing chemical formulas for ionic binary compounds. The activity consists of four parts:
- Students will write chemical formulas given a cation and an anion.
- Students will determine the charge of a transition metal given a formula of a compound. Students will write chemical formulas given the name of a compound.
- Students will name a compound given its formula.
- Reinforce understanding of the concept of ionic charges necessary to write the chemical formula of ionic compounds.
- Recognize that the valences of the elements determine the ratio of the elements in a compound
Vernier EasyData,Vernier EasyLink and Vernier EasyTemp are registered trademarks of Vernier Science Education.

