Interactive pH Titration
TI-Nspire™ CX CAS
Interactive pH Titration
In this lesson, students will explore an interactive titration curve for a weak acid-strong base titration. Students will learn how acid concentration, acid ionization constant, and base concentration affect the titration.
- Students will observe how the features of a weak acid-strong base titration curve change when varying the acid ionization constant and/or the concentrations of the weak acid and strong base.
- Students will determine an equation for the volume of base added at the equivalence point.
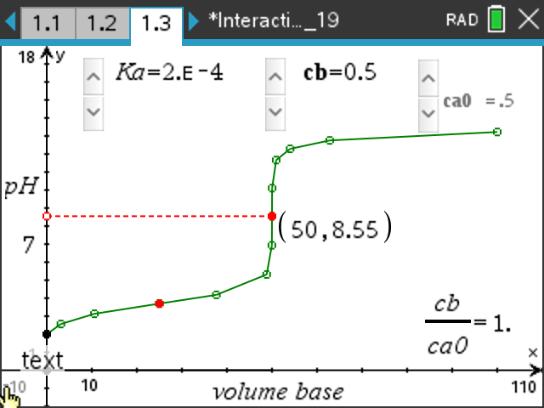
- Students will relate pH to pKa at the "halfway" point in a titration. They will see that pH changes slowly with volume of base in the region around the halfway point. This is the buffer region.
- acid ionization constant
- buffer
- equivalence point
- halfway point
- pH
- pKa
- titration curve
This lesson features an interactive titration curve for a weak acid-strong base titration.
As a result, students will have a better understanding of:
- How acid concentration, acid ionization constant, and base concentration affect the titration.
- The pH of a weak acid is not 7.0 at the equivalence point.
- The pH at the halfway point is equal to the pKa of the acid.
TI-Nspire™ CX CAS
Vernier EasyData,Vernier EasyLink and Vernier EasyTemp are registered trademarks of Vernier Science Education.

