Diffusion Data Collection Lab
- distilled water
- 0.1 M, 0.2 M, and 0.3 M salt solutions
- 400 mL or 500 mL beaker
- 25 mL or 50 mL graduated cylinder
- funnel
- four pieces of dialysis tubing approximately 10 cm long
- dental floss or string
Diffusion Data Collection Lab
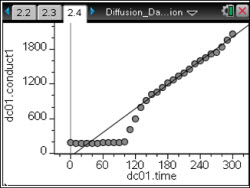
In this lesson, students will use varying molarities of a salt solution to observe diffusion through a membrane.
- Students will explore the movement of molecules through a membrane via diffusion.
- Students will explore the effects of concentration gradient on diffusion rate.
- diffusion
- concentration gradient
- molarity
- conductivity
- permeability
This activity involves students conducting 4 separate trials using 4 different molarities of a salt solution.
As a result, students will:- Use a conductivity probe to understand diffusion rates.
- Develop an understanding of the effect of the concentration of a solution on the rate of diffusion across a selectively permeable membrane.
You may want to have students create a Lab Report using TI-Nspire following this lab activity. A sample lab report is provided in the downloads.
- distilled water
- 0.1 M, 0.2 M, and 0.3 M salt solutions
- 400 mL or 500 mL beaker
- 25 mL or 50 mL graduated cylinder
- funnel
- four pieces of dialysis tubing approximately 10 cm long
- dental floss or string
Vernier EasyData,Vernier EasyLink and Vernier EasyTemp are registered trademarks of Vernier Science Education.

