Isotopes and Atomic Mass
Isotopes and Atomic Mass
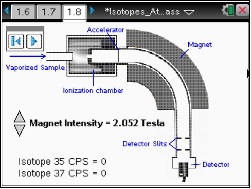
In this lesson, students will use a simulation of a mass spectrometer. Students will use a slider to adjust the magnetic field to direct either Cl-35 or Cl-37 to the detector.
- Students will run a mass spectrometer simulation to gain an understanding of how the masses and abundances of the isotopes are determined experimentally.
- Students will learn the relationship between the average atomic mass and the masses of the stable isotopes of that element.
- amu
- average atomic mass
- ionization
- isotope
- magnetic field
- mass spectrometer
- percent abundance
This lesson features a simulation of a mass spectrometer. Students will use a slider to adjust the magnetic field to direct either Cl-35 or Cl-37 to the detector.
In doing this activity students will:
- Gain an understanding of how the masses and abundances of the isotopes are determined experimentally.
- Practice doing problems involving isotopic masses and abundances and average atomic mass.
- Learn the relationship between the average atomic mass (as found in the Periodic Table) and the masses of the stable isotopes of that element.
This activity was created using Lua. Learn more about Lua in TI-Nspire here.
Vernier EasyData,Vernier EasyLink and Vernier EasyTemp are registered trademarks of Vernier Science Education.

