Balancing Chemical Equations - Practice
Balancing Chemical Equations - Practice
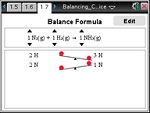
In this lesson, students use the ChemBox feature of TI-NspireTM technology to practice balancing chemical equations.
- Students will describe what “reactants” and “products” in a chemical equation mean.
- Students will explain the difference between “coefficients” and “subscripts” in chemical equations.
- Students will recognize that the number of atoms of each element is conserved in a chemical reaction.
- Students will balance a chemical equation.
- reactant
- synthesis (combination)
- product
- double replacement (precipitation)
- combustion
- neutralization (acid-base)
- decomposition
- single replacement (oxidation-reduction)
This lesson involves students using ChemBox feature of TI-NspireTM technology to practice balancing chemical equations.
- Students will use Edit mode of the activity to enter given reactants and products in a chemical equation.
- Students will use Balance mode for visual support necessary to balance a chemical equation.
As a result, students will:
- Reinforce understanding that the number of atoms is conserved in a chemical reaction
- Recognize the meaning of coefficients and subscripts in a chemical equation.
Vernier EasyData,Vernier EasyLink and Vernier EasyTemp are registered trademarks of Vernier Science Education.

