Forensics Case 9 - Killer Cup of Coffee: Using colorimetry to determine concentration of a poison
- This is Activity 9 from the EXPLORATIONS Book:
- Vernier EasyLink™
- Colorimeter
- 7 cuvettes
- Colored wax pencils
- Deionized or distilled water
- 50 mL of 0.15M ferrous thiocyanate solution
- 5 mL of ferrous thiocyanate solution with unknown concentration
- Two 10 mL pipettes or graduated cylinders
- Two 50 mL beakers
- 5 stirring rods
- 2 droppers
- 5 test tubes
- Test tube rack
- Lint-free tissues
- Waste beaker
- Goggles
Forensics: Connecting Science Investigations with TI Data Collection Activities.
The following materials are required for this activity:
Forensics Case 9 - Killer Cup of Coffee: Using colorimetry to determine concentration of a poison
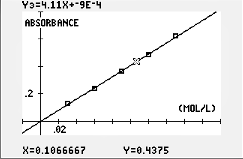
In this activity, students will use colorimetry to determine the concentration of a colored species in a solution and use a linear relationship to model Beer's law. They use Beer's law to determine the concentration of iron(III)thiocyanate (FeSCN2+) in an unknown solution.
- This is Activity 9 from the EXPLORATIONS Book:
- Vernier EasyLink™
- Colorimeter
- 7 cuvettes
- Colored wax pencils
- Deionized or distilled water
- 50 mL of 0.15M ferrous thiocyanate solution
- 5 mL of ferrous thiocyanate solution with unknown concentration
- Two 10 mL pipettes or graduated cylinders
- Two 50 mL beakers
- 5 stirring rods
- 2 droppers
- 5 test tubes
- Test tube rack
- Lint-free tissues
- Waste beaker
- Goggles
Forensics: Connecting Science Investigations with TI Data Collection Activities.
The following materials are required for this activity:
Vernier EasyData,Vernier EasyLink and Vernier EasyTemp are registered trademarks of Vernier Science Education.

