Waves and Spectrum Exploration
Waves and Spectrum Exploration
In this lesson, students will study the relationship between wavelength, frequency, and color through an interactive simulation.
- Students will study the relationship between wavelength, frequency, and color.
- Students will calculate the energy of electromagnetic radiation using Planck’s Constant.
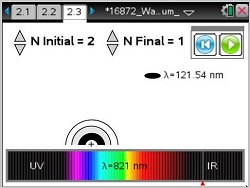
- Students will learn about the Balmer, Paschen, and Lyman Series.
- Students will calculate the energy change that an electron undergoes when changing energy levels using the Rhydberg Constant.
- speed of light
- wavelength
- frequency
- electromagnetic radiation
- visible spectrum
- photons
- Planck’s Constant
- Balmer Series
- Paschen Series
- Lyman Series
- Rhydberg Constant
This lesson involves the relationship between waves and electron energy jumps within the atom.
As a result, students will:
- Study the relationship between wavelength, frequency ,and color.
- Calculate wavelengths and frequencies.
- Calculate the energy of electromagnetic radiation using Planck’s Constant.
- Study the energy changes as electrons change energy levels in an atom.
- Learn about the Balmer, Paschen, and Lyman Series.
- Calculate the energy change that an electron undergoes when changing energy levels using the Rhydberg Constant.
Vernier EasyData,Vernier EasyLink and Vernier EasyTemp are registered trademarks of Vernier Science Education.

