Colligative Properties
Science: Chemistry: Solutions
Science: Chemistry: Energy and Matter
9-12
45 Minutes
2.1
Lessons
TNS
Colligative Properties
Activity Overview
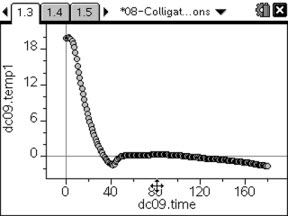
Investigate the freezing point depression due to different concentration of solute.
Objectives
About the Lesson
The following questions will guide student exploration during this activity:
In this activity, students first observe the freezing points of various aqueous sucrose solutions. They observe that the freezing point decreases as solute concentration increases. They use linear regression to estimate the freezing-point depression constant for water. Then, they use the accepted theoretical freezing-point depression constant to calculate the freezing points of various solutions.
Download Files
Science: Chemistry: Solutions
Science: Chemistry: Energy and Matter
9-12
45 Minutes
2.1
Lessons
TNS
iPad is a trademark of Apple Inc., registered in the U.S. and other countries.
Vernier EasyData,Vernier EasyLink and Vernier EasyTemp are registered trademarks of Vernier Science Education.
Vernier EasyData,Vernier EasyLink and Vernier EasyTemp are registered trademarks of Vernier Science Education.

